What is the AI-Powered Drug Formulation Market Size?
The global AI-powered drug formulation market is witnessing rapid growth as AI technologies enable pharmaceutical companies to design optimized drug formulations efficiently and accurately.The market growth is attributed to the increasing integration of advanced AI technologies in drug formulation processes, enabling faster development and reduced costs.
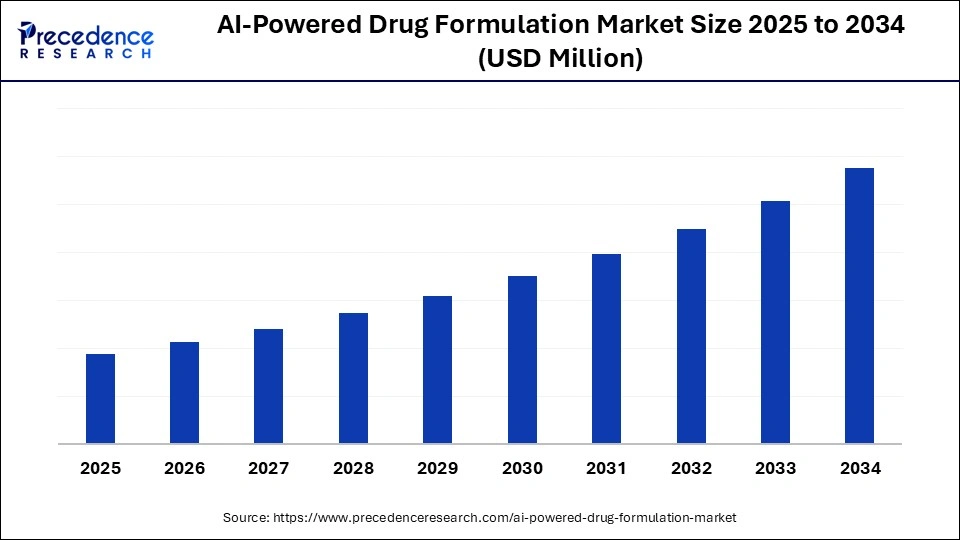
AI-Powered Drug Formulation Market Key Takeaways
- North America segment held a dominant presence in the market in 2024.
- The Asia Pacific segment is expected to grow at the fastest rate in the market during the forecast period of 2025 to 2034.
- By component, the software platforms segment accounted for a considerable share of the market in 2024.
- By component, the services segment is projected to experience the highest growth rate in the AI-powered drug formulation market between 2025 and 2034.
- By technology, the machine learning & predictive modelling segment led the market.
- By technology, the generative AI & hybrid AI approaches segment is set to experience the fastest rate of market growth from 2025 to 2034.
- By application/use case, the formulation design & optimisation segment registered its dominance over the market in 2024.
- By application/use case, the personalised & advanced therapy formulations segment is anticipated to grow with the highest CAGR in the market during the studied years.
- By drug type, the small molecules segment dominated the AI-powered drug formulation market in 2024.
- By drug type, the nucleic acid-based drugs and cell/gene therapy payloads segment is projected to expand rapidly in the market in the coming years.
- By dosage form, the oral solid dosage segment maintained a leading position in the market in 2024.
- By dosage form, the advanced delivery systems segment is predicted to witness significant growth in the market over the forecast period.
Significant Technological Change in the AI-Powered Drug Formulation Market
The drug formulation market is undergoing a transformative technological shift, driven by the need to develop drugs more quickly and adherence to regulatory requirements. Their solubility and pharmacokinetics. Notably, Insilico Medicine has developed generative chemistry platforms that can provide predictive formulation modeling, reducing experimental cycles and accelerating development timelines.
The other notable change is the merger of AI with continuous production and digital twins to produce drugs. Novartis or AstraZeneca, among others, are using AI-enabled digital twin models to recreate the formulation process. This enables adaptive production with a minimal percentage of waste and improved reproducibility. Additionally, AI enables real-time monitoring and optimization of formulation scale-up, allowing for enhanced yield and quality. These technological changes are making AI formulation a major force of innovation, efficiency, and competitiveness in the pharmaceutical sector.
What Is the Role of AI in Drug Formulation?
The growing need to develop drugs more quickly, with greater precision and at lower costs, is propelling the AI-powered drug formulation market. The field of pharmaceutical R&D is undergoing a revolution with the integration of AI technologies, including machine learning, deep learning, and computational chemistry. This facilitates the rapid design of formulations, predicting drug behaviour, and optimising dosage forms. These features reduce trial cycles, enhance effectiveness, and lower overall development costs.
Stanford University announced that AI-based simulation models shortened formulation design cycles by almost 40% on biologics projects in 2024. Large biopharma firms, including Pfizer, Novartis, and AstraZeneca, have adopted AI-based platforms into their drug development pipeline to speed up the preclinical development process. Furthermore, the development of AI-based predictive analytics in this market is also aiding in such expansions, as it is now possible to simulate the interactions between molecules and then test them physically.(Source: https://med.stanford.edu)
AI-Powered Drug Formulation Market Growth Factors
- Rising Adoption of AI in Early-Stage Drug Development: Growing advancements in AI algorithms are driving faster and more precise formulation predictions, boosting efficiency across R&D pipelines.
- Driving Data-Driven Formulation Strategies: Increasing access to high-quality biomedical datasets is propelling the integration of AI-powered predictive modelling into drug formulation processes.
- Growing Demand for Personalised Medicine: Rising need for tailored therapies is fuelling investment in AI platforms capable of optimising individualised drug formulations.
- Boosting Computational Drug Design Capabilities: Enhanced computing power and AI integration are accelerating simulation-driven drug formulation, reducing time-to-market for novel therapeutics.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Component, Technology, Application / Use Case, Drug Type, Dosage Form, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Trade & Investment Trends in the Global AI Powered Drug Formulation Market: Key Statistics and Developments
- The U.S. government alone committed USD 500 million through NIH and NSF initiatives to advance AI drug formulation capabilities. (Source: https://www.nsf.gov)
- By the end of 2024, North America accounted for over 62% of global AI-assisted drug formulation patents, led by Massachusetts and California, with strong contributions from MIT, Stanford, and UCSF. (Source: https://www.drugpatentwatch.com)
- The European Commission's Horizon Europe program dedicated €400 million in 2024 to AI-assisted drug formulation and manufacturing projects across member states, with an emphasis on sustainable and patient-specific drug delivery methods.
- In 2024, the Bill & Melinda Gates Foundation granted USD 120 million to initiatives integrating AI in drug formulation for neglected diseases, with projects involving collaborations between AI biotech startups and public health institutions.(Source:https://www.gatesfoundation.org)
- South Korea emerged as a significant AI drug formulation innovation centre in 2024, filing 45% more patents than in 2023, supported by KAIST and POSTECH's AI pharma programs. (Source: https://bioengineer.org)
- India's Department of Biotechnology (DBT) invested USD 200 million in AI-driven pharmaceutical formulation research in 2024, supporting over 60 projects involving complex dosage form optimisation. (Source: https://ehealth.eletsonline.com)
- In 2024, Japan allocated over USD 750 million to AI drug formulation research under its AMED program, focusing on reducing formulation cycle times and enhancing biologic drug delivery efficiency. (Source: https://pj.jiho.jp)
- In 2024, Stanford University and Pfizer launched a joint research centre dedicated to AI-based drug formulation, backed by USD 50 million in funding.(Source: https://www.prnewswire.com)
AI Powered Drug Formulation Market — Regulatory & Innovation Landscape
| Country / Region | Regulatory Body / Initiative | Key Regulations / Frameworks | Focus Areas | Notable Notes |
| United States | U.S. Food & Drug Administration (FDA) + NIH | FDA Digital Health Innovation Action Plan- 21st Century Cures Act (AI in clinical trials)- AI/ML Software as a Medical Device (SaMD) guidance | Regulatory validation of AI models, Data privacy compliance, Clinical trial automation | In 2024, the FDA launched the AI Drug Development Challenge to accelerate the development of AI-based formulation platforms. The NIH has funded over USD 250 million in AI-drug formulation projects. |
| European Union | European Medicines Agency (EMA) + Horizon Europe | EU AI Act (proposed)- Good Machine Learning Practice (GMLP)- MDR (Medical Device Regulation) | Ethical AI deployment, Transparency in AI-assisted trials, Data interoperability | Horizon Europe allocated €350 million in 2024 for AI-driven formulation research, with a focus on adaptive trial designs and formulation optimization. |
| China | National Medical Products Administration (NMPA) + Ministry of Science & Technology (MOST) | AI Drug Development Guidelines- Data Security Law- Pharmaceutical Data Standards (2023) | AI-driven formulation validation, Integration of AI in regulatory submissions, Standardised data pipelines | China announced a USD 1.2 billion AI-Pharma initiative in 2024 to build national AI formulation platforms, with strong integration with CAS research centers. |
| Japan | Pharmaceuticals and Medical Devices Agency (PMDA) + AMED | Digital Health Innovation Strategy- AI Verification Guidelines for Medical Devices | AI-assisted drug formulation evaluation, Regulatory sandbox for AI validation, Biologics formulation AI | In 2024, PMDA expanded the regulatory sandbox for AI formulation trials to reduce approval timelines. |
| India | Central Drugs Standard Control Organisation (CDSCO) + DBT + CSIR | Draft National AI Strategy for Healthcare- Bioinformatics & Computational Chemistry Standards- Clinical Trial Regulation Updates | AI model certification sharing for drug formulation, Ethical AI use in pharma | DBT funded over 50 AI-drug formulation projects in 2024, emphasizing dosage optimization for generics and complex biologics. |
| South Korea | Ministry of Food and Drug Safety (MFDS) + KRIBB | AI Based Drug Development Guidelines- Good Clinical Data Practice (GCDP)- AI model transparency requirements | AI-assisted biologics formulation, Clinical simulation platforms, Regulatory model certification | KAIST and POSTECH reported breakthroughs in AI formulation in 2024, reducing biologics formulation timelines. |
(Source:https://www.fda.gov)
(Source: https://digital-strategy.ec.europa.eu)
(Source: https://www.drugpatentwatch.com)
(Source: https://www.pmda.go.jp)
Market Dynamics
Drivers
How is the growing demand for data-driven formulation design reshaping the AI-Powered Drug Formulation Market?
The increasing demand for data-driven formulation design is expected to drive market growth in the coming years. Researchers and formulation scientists increasingly use deep learning and other sophisticated analytics to refine chemical, biological, and clinical information. Predictive models applied in molecular screening, solubility prediction, and excipient compatibility enable developers to acquire a more accurate formulation with fewer laboratory experiments. (Source: https://jamanetwork.com)
Increasing evidence in the year 2024 indicates that the integration of smart discovery instruments can have a significant impact on formulation development. An article published in JAMA states that 164 AI-related platforms are in clinical development in 2024, compared to 19 in 2023. Furthermore, the growing emphasis on reducing formulation development time and costs is expected to drive demand for AI-integrated formulation solutions.(Source:https://www.iqvia.com)
High upfront costs for AI infrastructure, high-performance computing, and integration of digital laboratories are expected to restrain the market. This cost constraint reduces the rate of adoption of intelligent formulation systems by new entrants and smaller players. Additionally, the stringent regulations surrounding patient data and pharmaceutical research are projected to restrain market expansion.
Opportunity
How does rising investment in digital transformation drive innovation in the AI-Powered Drug Formulation Market?
Growing investment in digital transformation of pharmaceutical R&D is anticipated to create immense opportunities for the AI-powered drug formulation market. Incorporating AI learning infrastructures and digital labs, major manufacturing pharmaceutical firms and research institutions are investing significant budgets in these fields. These investments facilitate automated experimentation, testing using simulations, and high-level formulation optimisation models.
Digital R&D financial and strategic investments hasten product development streams and strengthen competitive differentiation in the pharmaceutical ecosystem. A 2024 R&D report by IQVIA stated that more than 40% of late-stage drug development programs currently use AI-based formulation and predictive modelling tools, compared to 27% in 2022. Furthermore, the increasing use of advanced simulation and modeling techniques is likely to enhance the integration of artificial intelligence in formulation science.(Source: https://www.iqvia.com)
Segment Insights
Component Insight
How Are Software Platforms Driving the Core Transformation of the AI-Powered Drug Formulation Market?
The software segment dominated the AI-powered drug formulation market in 2024, as formulation development became a highly data-driven process. In 2024, the EFPIA reported a yearly increase in the use of AI-enabled software for creating drug formulations across Europe.
This resurgence is indicative of increasing penetration of artificial intelligence-based chemical simulation, process automation, and prediction capacity into formulation pipelines. Furthermore, the major providers of AI platform offerings, including IBM Watson Health and Google DeepMind, have begun to incorporate quantum computing techniques into their offerings, enabling them to make molecular predictions more quickly.(Source: https://efpia.eu)
The service segment is expected to grow at the fastest rate in the coming years, owing to the increasing industry trend of outsourcing the complexity of formulation. A 2024 report by the OHE indicates that more than 35% of mid-sized biopharma companies in North America and Europe. They have already undertaken AI-driven contract formulation projects to speed up time-to-market, thereby further boosting the market.(Source: https://www.biocon.com)
Technology Insights
How Are Machine Learning and Predictive Modelling Technologies Powering Innovation Across the Market?
The machine learning and predictive modeling segment held the largest revenue share in the AI-powered drug formulation market in 2024, due to its ability to process large volumes of data and provide accurate predictions about the formulation. These technologies have played a key role in improving accuracy in formulation and accelerating development processes, further supporting their shift to become the predominant technology in the market.
The generative AI and hybrid AI strategies segment is expected to grow at the fastest rate in the coming years, as they can develop new formulations with more efficiency than ever before.
In 2024, research at the Francis Crick Institute and ETH Zurich emphasised how generative AI can cut the number of formulation design cycles. Furthermore, the hybrid AI, a type of AI that combines machine learning and deep learning, provides a more accurate model of complex molecules and biologics, with significantly greater predictive power. (Source: https://ethz.ch)
Application / Use Case Insights
How Is AI Revolutionizing Formulation Design and Optimization Across Multiple Drug Delivery Platforms?
The formulation design and optimisation segment dominated the AI-powered drug formulation market in 2024, as drug development is becoming more complex and requires more efficient operations. Additionally, the use of AI in formulation design is likely to continue increasing as pharmaceutical companies strive to improve the efficiency and effectiveness of their drug development pipeline.
The personalized & advanced therapy formulations segment is expected to grow at the fastest CAGR in the coming years. Owing to the rising demand for treatments that would address the needs of a specific patient profile, such as genetic composition, disease nature, and environmental forces. Furthermore, the introduction of advanced smart nanotechnology and 3D printing is further propelling the personalized medicine sphere, resulting in superior and more precise treatments.
Drug Type Insights
What Makes Small-Molecule Formulations the Cornerstone of the AI-Powered Drug Formulation Ecosystem?
The small molecules segment held the largest revenue share in the AI-powered drug formulation market in 2024, as it has a well-established position in therapeutic development and is embedded with AI technology to enhance its effectiveness and safety profiles. Additionally, such a concerted effort marks the industry's willingness to utilize AI to optimize the small molecule drug development process, thereby facilitating segment dominance in the market.
The nucleic acid-based drugs and cell/gene therapy payloads segment is expected to grow at the fastest rate in the coming years, owing to the rising number of genetic disorders and the development of new technology of gene-editing technologies. This provides a new opening in the treatment of diseases. Moreover, AI finds its application in the creation of cell and gene therapies, analyzing enormous amounts of genomic data to reveal potential therapeutic targets and predict patient-specific responses.
Dosage Form Insights
Why Do Oral Solid Dosage Formulations Continue to Lead AI-Powered Formulation Research and Development?
The oral solid dosage segment dominated the AI-powered drug formulation market in 2024, as it is relatively affordable, simple to administer, and widely accepted by patients. In 2024, the Office for Health Economics (OHE) conducted a study that reported a 27% increase in the efficiency of formulating oral solid dosage products through the use of AI predictive modeling.
The Schrodinger and BenevolentAI platforms, based on AI, have improved the design of oral solid dosages by predicting the best excipient mixtures and dissolution profiles, thereby reducing the number of trial experiments. Additionally, the combination of real-world and experimental data was enhanced by collaboration between AI developers (XtalPi) and pharmaceutical giants, which also contributes to the prevalence of oral solid dosage forms.(Source: https://bmcmedinformdecismak.biomedcentral.com)
The Advanced Delivery Systems segment is expected to grow at the fastest rate in the coming years, owing to its ability to enhance drug solubility, targeted delivery, and controlled release. LNPs, which are being used in mRNA vaccines, including those developed by Moderna and BioNTech, have shown a high clinical success rate, and further AI-led progress in this area is encouraged. Furthermore, the growth in this sub-segment is also supported by industry alliances, such as the partnership between Pfizer and Arzeda to design AI-enhanced LNP.
Regional Insights
Why did North America Dominate the AI-powered drug formulation market?
North America led the AI-powered drug formulation market, capturing the largest revenue share in 2024, due to its robust infrastructure, substantial funding for research, and favorable regulatory systems. In 2024, the U.S. Department of Health and Human Services plans to expand its AI-for-Drug Development Program through multi-institutional projects, focusing on formulation efficiency and the development of drugs for rare diseases.
This was also accompanied by the efforts of the National Science Foundation and NIH to incorporate AI attributes into pharmaceutical research pipelines. Boston, San Francisco, and San Diego, which are the hubs, reported an influx of AI-driven projects. Furthermore, this form of innovation is redefining the way formulations are developed, tested, and commercialized in the region and establishing a leading role for North America.(Source: https://www.fda.gov)
The Asia Pacific is anticipated to grow at the fastest rate in the market during the forecast period, driven by substantial government efforts, increased biotech investments, and rapid adoption of AI. In 2024, the Ministry of Science and Technology in China introduced its AI Pharma Leap program to fund AI-based drug formulation, with a particular focus on biologics and treatments for rare diseases. (Source: https://www.navlindaily.com)
This involved collaboration with Tsinghua University, Peking University, and leading pharma companies to create proprietary AI platforms. In 2024, Seoul National University collaborated with Samsung Biologics to roll out next-generation AI formulation systems. This predicts stability data of complex biologics before physical testing, thus further facilitating the market growth in the coming years.(Source:https://samsungbiologics.com)
Top Vendors in AI Powered Drug Formulation Market & Their Offerings
- Pfizer Inc. (USA): A global biopharmaceutical leader, Pfizer integrates AI-driven formulation tools to accelerate drug discovery and optimise dosage forms.
- Johnson & Johnson (USA): This healthcare giant employs AI in drug formulation to enhance clinical trial efficiency and design personalised medicines.
- Novartis AG (Switzerland): Novartis leverages AI-powered platforms to improve formulation development for complex therapies.
- Roche Holding AG (Switzerland): Roche focuses on AI-driven drug formulation for biologics, using machine learning to optimise pharmacokinetics and enhance formulation efficacy.
- Merck & Co., Inc. (USA): Merck employs AI in predictive formulation modelling, accelerating early-stage development.
- AstraZeneca plc (UK): AstraZeneca integrates AI into its formulation research, particularly for inhalable biologics and oncology therapeutics.
- GlaxoSmithKline plc (UK): GSK utilizes AI in drug formulation to enhance stability, solubility, and bioavailability.
AI-Powered Drug Formulation Market Companies
- Exscientia: A global leader in AI-driven drug design and formulation, Exscientia integrates automated laboratories and predictive modeling to optimize compound properties early in development. The company has successfully advanced multiple AI-designed molecules into clinical trials, particularly in oncology and inflammation.
- Insilico Medicine: utilizes deep generative AI to design novel drug molecules and optimize their formulation profiles for enhanced bioavailability and stability. Its platforms Chemistry42 and PandaOmics enable end-to-end discovery and formulation, cutting development time from years to months.
- Atomwise: Atomwise leverages deep learning-based structure prediction through its AtomNet platform to enhance drug formulation and stability predictions. It collaborates with global pharma and biotech companies to accelerate formulation design for challenging small molecules.
- Schrödinger: Schrödinger combines AI with physics-based computational chemistry to optimize drug solubility, stability, and formulation compatibility. Pharmaceutical companies widely use its modeling platform for structure-guided formulation and molecule refinement.
- BenevolentAI: UK-based BenevolentAI applies machine learning and data mining to optimize drug formulation, focusing on target identification and molecular property tuning. The company's AI platform integrates data from chemistry, biology, and pharmacology to inform formulation decisions in complex therapeutic areas.
Other Key Players in the AI-Powered Drug Formulation Market
- Cyclica (part of Recursion): Uses AI to model multi-target drug interactions and optimize molecular profiles for stable formulations.
- DeepMatter: Provides digital chemistry and AI tools to improve reproducibility and precision in formulation development.
- IBM Watson Health: Partners with pharma companies to integrate AI in drug formulation optimization and process design.
- Standigm: Focuses on AI-driven formulation prediction and drug repurposing, primarily for CNS and metabolic disorders.
- Valo Health: Uses its Opal platform to integrate multi-omic data and AI for predictive formulation and pharmacokinetic modeling.
Recent Developments
- In September 2025, Eli Lilly announced the launch of a next-generation artificial intelligence and machine learning platform that enables biotechnology companies to access advanced drug discovery models built from years of the company's proprietary research data. The initiative reflects a growing industry trend toward adopting AI-driven approaches for drug discovery and safety testing. The move aligns with the U.S. FDA's ongoing push to minimise animal testing and accelerate preclinical research through digital and data-driven alternatives.
- In May 2025, following the completion of its USD 12 million Series A financing round, Persist AI introduced its Cloud Lab platform—an AI-powered system designed to accelerate pharmaceutical formulation development while reducing material consumption. The platform combines predictive AI modelling with automated robotic systems to generate, build, and test formulation prototypes, significantly improving formulation accuracy and efficiency across early-stage R&D pipelines.
(Source: https://www.reuters.com)
(Source: https://manufacturingchemist.com)
(Source: https://www.prnewswire.com)
(Source: https://www.digitalhealthnews.com)
Segments Covered in the Report
By Component
- Software Platforms
- AI/ML formulation design platforms
- Cloud-based formulation simulation tools
- Predictive analytics for excipient compatibility
- Services
- AI-enabled formulation-as-a-service (FaaS)
- Contract formulation support with AI integration
- Hardware & Infrastructure
- High-performance computing (HPC) clusters
- Laboratory automation & robotics integrated with AI
- IoT-enabled lab instruments
By Technology
- Machine Learning & Deep Learning
- Natural Language Processing (NLP) for literature mining
- Predictive Modeling & Simulation
- Generative AI & Neural Networks (novel formulation design)
- Reinforcement Learning (process optimization)
- Hybrid AI (AI + mechanistic models)
By Application / Use Case
- Excipient Compatibility Prediction
- Formulation Design & Optimization (IR, MR, ODT, biologics formulations)
- Stability & Shelf-life Prediction
- Bioavailability & Pharmacokinetic (PK) Simulation
- High-Throughput Screening (HTS) Optimization
- Personalized & Precision Medicine Formulations
- Advanced Therapy Formulations (cell/gene therapy delivery)
By Drug Type
- Small Molecules
- Biologics (proteins, peptides, mAbs)
- Nucleic Acid-Based Drugs (siRNA, mRNA, DNA)
- Cell & Gene Therapy Payloads
- Vaccines (traditional and mRNA-based)
By Dosage Form
- Oral Solid Dosage (tablets, capsules)
- Parenterals (injectables, biologics formulations)
- Inhalation & Pulmonary Drug Delivery
- Transdermal / Topical Formulations
- Nasal / Mucosal Delivery
- Advanced Delivery Systems (liposomes, nanoparticles, LNPs)
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting