Brugada Syndrome Market Size and Forecast 2025 to 2034
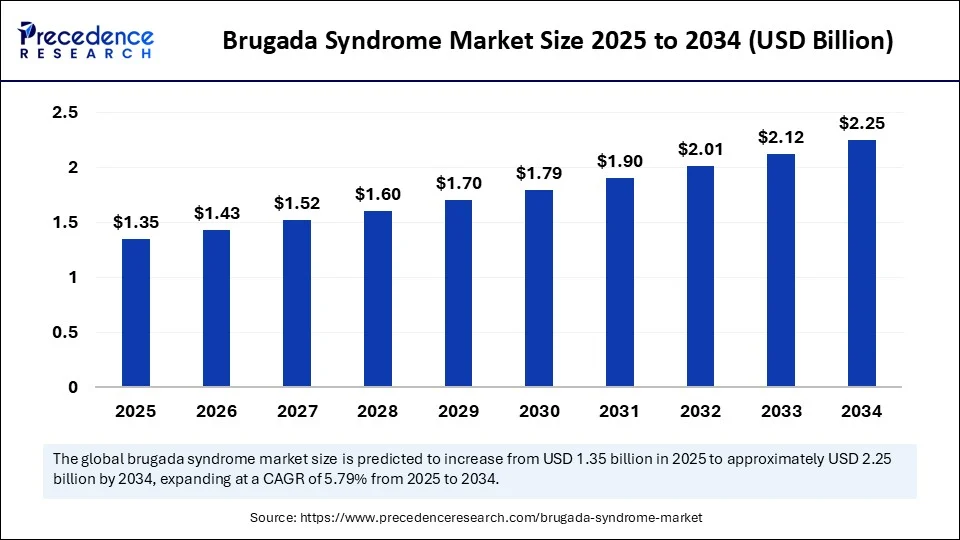
The global brugada syndrome market size accounted for USD 1.28 billion in 2024 and is predicted to increase from USD 1.35 billion in 2025 to approximately USD 2.25 billion by 2034, expanding at a CAGR of 5.79% from 2025 to 2034. The increased disease awareness, advancements in diagnostics, and growing emphasis on personalized care are expected to boost the growth of the market during the forecast period.
Brugada Syndrome Market Key Takeaways
- In terms of revenue, the global brugada syndrome market was valued at USD 1.28 billion in 2024.
- It is projected to reach USD 2.25 billion by 2034.
- The market is expected to grow at a CAGR of 5.79% from 2025 to 2034.
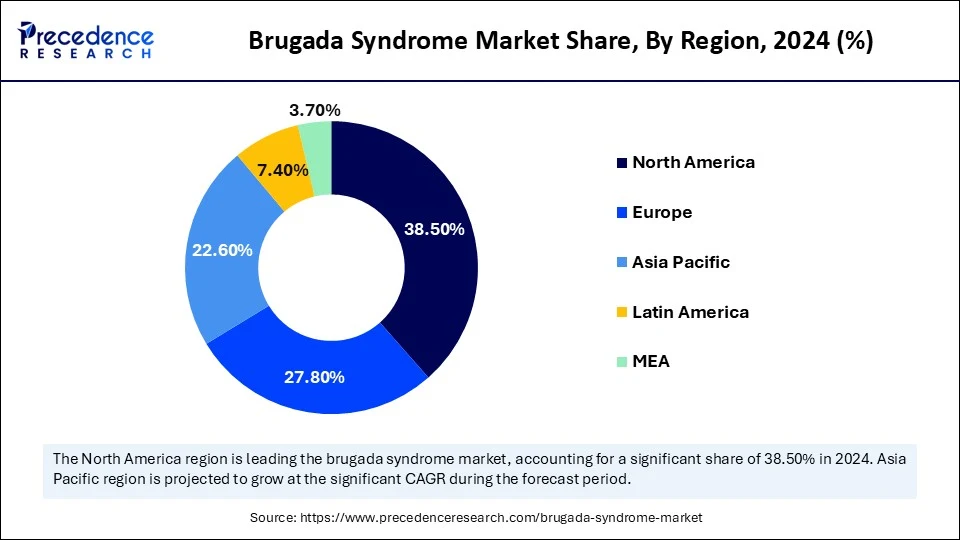
- North America dominated the brugada syndrome market with the largest market share of 38.5% in 2024.
- Asia Pacific is expected to expand at the fastest CAGR between 2025 and 2034.
- By type of treatment, the implantable devices segment led the market, under which the implantable cardioverter defibrillators (ICDs) sub-segment held the largest share of 41.2% in 2024.
- By type of treatment, the drug therapy segment is expected to grow at a remarkable CAGR between 2025 and 2034.
- By diagnosis & monitoring, the electrocardiogram (ECG) segment dominated the market, under which the resting ECG segment held the largest share of 48.7% in 2024.
- By diagnosis & monitoring, the genetic testing segment is expected to grow at a remarkable CAGR between 2025 and 2034.
- By end-user, the hospitals segment held the largest market share of 52.3% in 2024.
- By end-user, the cardiology clinics segment is expected to grow at a remarkable CAGR between 2025 and 2034.
- By distribution channel, the hospital pharmacies segment contributed the highest market share of 46.9% in 2024.
- By distribution channel, the online pharmacies segment is expected to grow at a remarkable CAGR between 2025 and 2034.
How is AI Revolutionizing the Brugada Syndrome Market?
Artificial intelligence (AI) is transforming the Brugada syndrome market by enhancing early detection and clinical decision-making. Machine learning models trained on ECG data can now identify subtle electrical abnormalities associated with Brugada syndrome that might otherwise go unnoticed by traditional methods. AI-powered diagnostic tools are helping clinicians reduce misdiagnosis rates and stratify patients' risk more accurately. Predictive analytics also aid in identifying candidates for implantable cardioverter-defibrillation, a life-saving intervention. Moreover, AI is accelerating drug discovery by simulating responses to anti-arrhythmic drugs in virtual cardiac models. As precision medicine evolves, AI's role in Brugada syndrome care is set to deepen, enabling proactive and personalized interventions.
U.S. Brugada Syndrome Market Size and Growth 2025 to 2034
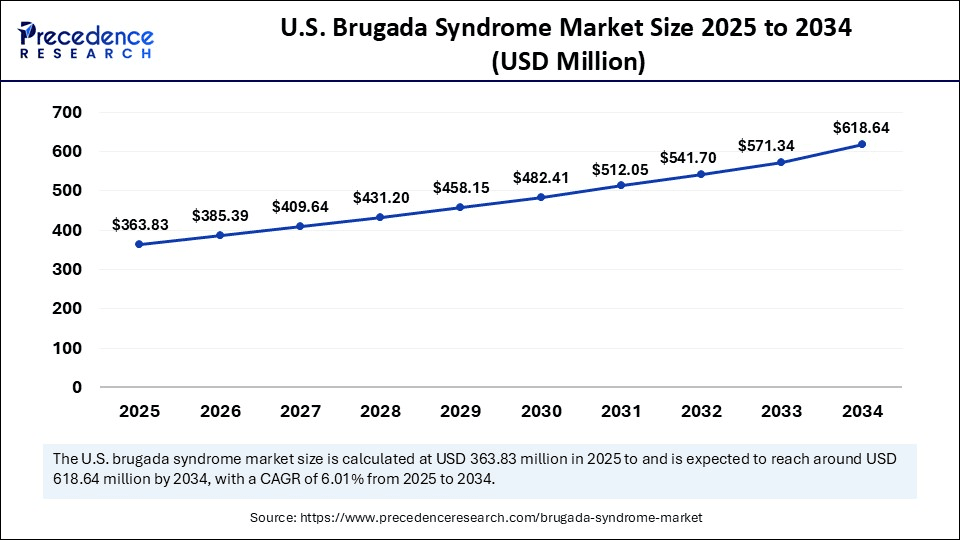
The U.S. brugada syndrome market size was exhibited at USD 344.96 million in 2024 and is projected to be worth around USD 618.64 million by 2034, growing at a CAGR of 6.01% from 2025 to 2034.
What Made North America the Dominant Region in the Brugada Syndrome Market in 2024?
North America dominated the market with the largest share in 2024, due to its advanced healthcare infrastructure and early adoption of cutting-edge diagnostics. The region benefits from a higher level of awareness among healthcare providers, which contributes to faster diagnosis and management. Research institutions across the U.S. and Canada are actively involved in Brugada-focused clinical trials and genetic research. Availability of implantable cardioverter defibrillators and reimbursement support further encourage timely treatment. Collaborations between academic institutions and industry players are accelerating innovation. North America's favorable regulatory environment also supports the rapid development of therapies for rare conditions, such as Brugada syndrome.
The U.S. is a major player in the market. The growth of the market in the U.S. is driven by increased research funding. Major hospitals and cardiac centers in the U.S. are equipped with advanced ECG mapping systems and offer widespread access to ICDs. The country is also home to biotech companies that are actively developing targeted therapies and AI-driven diagnostic platforms. Federal initiatives supporting rare disease research contribute to improved awareness and innovation. Additionally, the U.S. healthcare system supports a growing number of genetic counselling services, enhancing early identification of at-risk individuals. The strong ecosystem of R&D, clinical care, and regulatory support makes the U.S. a global leader in this field.
What Factors Contribute to the Growth of the Asia Pacific Brugada Syndrome Market?
Asia Pacific is projected to be the fastest-growing market for Brugada syndrome, driven by ongoing improvements in healthcare infrastructure and the early adoption of innovative diagnostic technologies. The region benefits from increased awareness among healthcare professionals, enabling quicker diagnosis and management. Asia Pacific is contributing significantly through the expansion of healthcare capabilities and investment in research. The growing presence of cutting-edge medical devices and increased cooperation between academic institutions and industry further support innovation and improved patient outcomes.
China is a key player in the Brugada syndrome market in Asia Pacific, due to its strong investments in healthcare modernization and cardiac research. The government's commitment to expanding genetic testing and promoting early disease detection is creating a conducive environment for growth. Leading hospitals in China are adopting AI-driven ECG systems and incorporating Brugada screening into routine cardiovascular care. Additionally, Chinese medical device manufacturers are developing and domesticating ICD technologies, reducing costs and increasing accessibility. National initiatives such as the Healthy China 2030 policy prioritize cardiovascular health, further supporting Brugada-related programs. With a large population and improving diagnostics capabilities, China is emerging as a hub for advanced cardiac care.
Market Overview
Brugada syndrome is a rare but potentially fatal genetic arrhythmia disorder characterized by abnormal electrocardiogram (ECG) findings and an increased risk of sudden cardiac death due to ventricular fibrillation. The condition is caused by mutations, most notably in the SCN5A gene, leading to dysfunction in cardiac sodium ion channels. While many patients remain asymptomatic, others experience syncope, palpitations, or sudden cardiac arrest. The market for Brugada Syndrome includes diagnostic tools, pharmacological treatments, implantable devices, genetic testing, and supportive monitoring technologies.
The Brugada syndrome market is expanding steadily, driven by technological advancements in diagnostics, improved genetic screening, and rising awareness among cardiologists and emergency care professionals. While the disease remains underdiagnosed in many regions, increased availability of advanced ECG interpretation tools is supporting early intervention. Implantable devices, such as ICDs, remain the cornerstone of treatment, while research into pharmacological approaches continues. Market growth is also bolstered by academic collaborations and grants that support research on rare diseases. With sudden cardiac death among young adults drawing global attention, efforts to improve the detection and treatment of Brugada syndrome are intensifying. This heightened focus is translating into more funding, innovation, and global access to care.
Market Trends
- Increased integration of AI and wearable ECG devices for continuous rhythm monitoring.
- Growing emphasis on genetic testing for familial screening and early detection.
- Development of novel anti-arrhythmic therapies tailored to Brugada syndrome.
- Expansion of public and private investment in rare cardiac disease research and awareness campaigns.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 2.25 Billion |
| Market Size in 2025 | USD 1.35 Billion |
| Market Size in 2024 | USD 1.28 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 5.79% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type of Treatment, Diagnosis & Monitoring, End User, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
What is Driving the Growth of the Brugada Syndrome Market?
One of the key drivers of the Brugada syndrome market is the growing accessibility to genetic testing. As more individuals seek clarity on inherited cardiac conditions, demand for genetic screening tools continues to rise. Genetic testing helps identify individuals at risk early, even before symptoms appear. Additionally, advancements in ECG technologies and mobile health monitoring devices are facilitating early diagnosis. Government initiatives that support rare disease awareness and innovation also play a significant role. Hospitals and cardiology clinics are investing in training and technologies to better diagnose and manage this condition. The combination of genetic insights and medical tech is pushing the market forward.
Restraint
Rarity of the Condition and High Cost of Advanced Testing
Despite advancements, the Brugada syndrome market faces several restraints. The disease often presents with subtle or no symptoms, leading to underdiagnosis or misdiagnosis, especially in emergency settings. A lack of awareness among general practitioners and even some cardiologists delays timely intervention. The high cost and limited availability of advanced electrophysiological testing and ICDs in low-income regions hinder equitable access. Moreover, reimbursement challenges for genetic testing and ICD procedures add financial strain. Inconsistency in clinical guidance across countries also affects the uniformity of care. Bridging these gaps will be crucial for market expansion and patient safety.
Opportunity
Personalized Care is the Next Frontier
The market holds significant untapped potential in personalized medicine and targeted therapies. There is a growing need for patient-specific treatment plans based on genetic profiles and electrophysiology offer opportunities for digital diagnostic platforms. Moreover, pharmaceutical companies are investing in next-generation anti-arrhythmic drugs designed to stabilize the heart's electrical activity without the need for invasive procedures. Regulatory fast-tracking of orphan disease treatments is also opening avenues for quicker product development. With increasing attention from biotech firms, the Brugada syndrome market is ripe for innovation.
Type of Treatment Insights
Why Did the Implantable Devices Segment Dominate the Brugada Syndrome Market in 2024?
The implantable devices segment dominated the market, under which the implantable cardioverter defibrillators (ICDs) sub-segment held the largest share in 2024. This is primarily due to their proven effectiveness and lifesaving capabilities. They are considered the gold standard for patients at high risk of sudden cardiac death, particularly those with a history of ventricular fibrillation or syncope. ICDs continuously monitor the heart rhythm and deliver a shock if a life-threatening arrhythmia is detected. Their effectiveness in preventing cardiac arrest has made them the preferred choice among cardiologists.
The growing awareness of sudden cardiac arrest in otherwise healthy individuals has increased demand for ICD implementation. Advancements in device miniaturization, battery longevity, and remote monitoring have enhanced patient compliance. Healthcare systems in developed through reimbursement schemes. Moreover, ICDs are often used in conjunction with drug therapy, improving overall outcomes. As more electrophysiology centers are established, the adoption of ICDs is accelerating globally. Despite cost constraints in emerging markets, the use of these products continues to grow in high-risk populations.
New-generation ICDs are also being developed with features such as dual-chamber pacing and wireless communication, enabling better follow-up and integration with AI diagnostics. These innovations help reduce inappropriate shocks and enhance patient quality of life. Education programs are also helping patients and caregivers understand the function and benefits of devices. Clinical guidelines continue to prioritize ICDs for primary prevention in Brugada syndrome patients with documented arrhythmias. With long-term survival data reinforcing their utility, ICDs remain central to the market landscape. Continued R&D ensures that this segment will maintain dominance for years to come.
The drug therapy segment is expected to grow at the fastest rate, with the quinidine drug therapy sub-segment leading the charge during the forecast period due to its effectiveness in preventing ventricular arrhythmias in Brugada patients. It is especially valuable for individuals who are not ideal candidates for ICDs or prefer non-invasive approaches. Quinidine works by inhibiting the transient outward potassium current, stabilizing cardiac action potentials. With rising focus on personalized and pharmacological treatment options, Quinidine is experiencing renewed interest. Its ability to reduce electrical heterogeneity in the heart makes it a critical tool in arrhythmia management. This niche therapy is expanding its footprint among clinicians seeking alternatives to surgery.
Patients' ineligible for device implantation due to age comorbidities, or psychological reasons often turn to Quinidine as a viable option. Moreover, its role in the emergency arrhythmic storm market makes it an important drug for acute care. As electrophysiologists become more familiar with its use in Brugada syndrome, prescriptions are rising. Clinical studies showing promising results in arrhythmia suppression are supporting its reintroduction in modern treatment protocols. There is also growing interest in low-dose, sustained-release versions for long-term use, which is expected to boost demand for Quinidine.
It is especially favored in patients with spontaneous type 1 ECG patterns. For individuals not yet eligible for ICDs, it serves as a primary management tool. Its versatility in both acute and long-term settings makes it a mainstay of Brugada drug therapy. Cardiologists prefer Quinidine for its targeted action and broad safety profile when monitored properly.
The isoproterenol sub-segment is expected to experience steady growth in the upcoming period, driven by its high usage for acute arrhythmic storms and drug-induced ECG abnormalities. It works by stimulating adrenergic receptors, particularly in cases of acute arrhythmic storms and drug-induced ECG abnormalities. It works by stimulating adrenergic receptors to increase heart rate and cardiac output, making it highly effective in emergencies. This medication is often used in the treatment of bradycardia and heart block, where it helps restore normal heart rhythm. Its rapid onset of action and predictable effects make it a preferred choice for clinicians in critical care settings. Furthermore, advancements in drug delivery systems have enhanced their efficacy and safety profile.
Diagnosis & Monitoring Insights
What Made Electrocardiogram (ECG) the Dominant Segment in the Market?
The electrocardiogram (ECG) segment dominated the Brugada syndrome market, under which the resting ECG segment held the largest share in 2024. The segment's dominance stems from its critical role in identifying the Type 1 Brugada ECG pattern, especially in symptomatic patients. As the first step in most diagnostic pathways, a resting ECG is performed in emergency departments, clinics, and screening programs. Its cost-effectiveness and widespread accessibility make it indispensable, even in low-resource settings. Continuous improvements in ECG machines and interpretation algorithms are further supporting its use. For cardiologists and general practitioners alike, resting ECG remains the foundation of the initial Brugada assessment.
The segment's dominance also reinforced by the fact that many Brugada cases are diagnosed incidentally during routine ECGs or pre-surgical screenings. Resting ECG is crucial not only for initial detection but also for ongoing patient monitoring and response to treatment. In high-risk patients, periodic ECGs help assess disease progression and therapeutic impact. Digital ECG systems with AI-powered pattern recognition are making resting ECGs more accurate and efficient.
The genetic testing segment is expected to experience the fastest growth in the market during the forecast period. The SCN5A genetic testing sub-segment is a leading diagnostic approach in Brugada syndrome care due to its precision in identifying pathogenic mutations. As Brugada is an inherited arrhythmic condition, identifying mutations in the SCN5A gene helps confirm the diagnosis and assess familial risk. This form of testing is increasingly being integrated into clinical pathways, particularly for asymptomatic individuals with a family history of sudden cardiac death. Genetic results guide treatment decisions, including the need for ICDs or pharmacologic therapy. Advancements in next-generation sequencing have made SCN5A testing more accessible and affordable. These factors are driving the rapid adoption of technology across global healthcare systems.
Genetic testing also supports the broader goal of personalized medicine by enabling tailored risk assessments. As the understanding of genotype-phenotype relationships evolves, SCN5A mutation carriers can be more accurately stratified into high- or low-risk categories. Hospitals are incorporating genetic counselors to help families understand and interpret results and plan preventive care. Regulatory bodies are encouraging genetic screening as part of national strategies for addressing rare diseases. In pediatric and neonatal cardiology, SCN5A testing is gaining traction for early intervention and diagnosis. Its ability to detect silent carriers before symptoms manifest adds a powerful layer to public health initiatives.
End-User Insights
Why Did the Hospitals Segment Dominate the Brugada Syndrome Market in 2024?
The hospitals segment dominated the market in 2024 due to their comprehensive diagnostics and treatment capabilities. These institutions are equipped with ECGs, electrophysiology labs, genetic testing facilities, and cardiac ICUs, enabling them to manage both acute and chronic Brugada cases. Most ICD implementations and drug trials take place in hospital settings under the supervision of trained electrophysiologists. Emergency departments are also key access points for initial diagnosis in patients presenting with arrhythmic episodes. Hospitals serve as central hubs for patient follow-up, monitoring, and education. Their integrated infrastructure supports end-to-end care, reinforcing their dominance.
The cardiology clinics segment is expected to experience the fastest growth in the market during the forecast period, due to their specialized expertise and focus on personalized cardiac care. These clinics are often the first point of contact for patients with unexplained syncope, palpitations, or abnormal ECG findings. Equipped with advanced diagnostics tools such as high-resolution ECGs and holter monitors, cardiology clinics are well-positioned to detect subtle Brugada patterns. They also serve as hubs for continuous monitoring, second opinions, and long-term management of asymptomatic or borderline cases. The growing referral network between general practitioners and cardiologists is further driving patient traffic to these centers.
Distribution Channel Insights
How Does the Hospital Pharmacies Segment Dominate the Market in 2024?
The hospital pharmacies segment dominated the Brugada syndrome market in 2024, due to their accessibility, trust, and wide geographic coverage. Patients benefit from face-to-face pharmacist consultations, which aid adherence and proper usage. These outlets often handle both branded and generic drugs efficiently, offering flexible purchasing options. Insurance coverage and established supply chains further support their dominance. Hospital pharmacies also cater to patients who prefer traditional buying methods over digital platforms. High prescription volumes and in-store promotional efforts reinforce their continued dominance.
On the other hand, the online pharmacies segment is expected to grow at the fastest rate in the upcoming period, driven by convenience, privacy, and the increasing adoption of telehealth. Patients can order medication discreetly, compare prices, and access virtual consultations all from the comfort of their own homes. This is especially appealing for weight loss treatments, where concerns about stigma or confidentiality may exist. Improved cold-chain logistics and fast shipping are making online purchases more reliable. Regulatory clarity and digital prescriptions are enhancing consumer confidence in this channel. As e-commerce continues to reshape healthcare access, online pharmacies are becoming an essential player in the Brugada syndrome market.
Brugada Syndrome Market Companies

- Medtronic plc
- Abbott Laboratories
- Boston Scientific Corporation
- Biotronik SE & Co. KG
- MicroPort Scientific Corporation
- Nihon Kohden Corporation
- GE HealthCare
- Koninklijke Philips N.V.
- Johnson & Johnson (Biosense Webster)
- OSYPKA Medical GmbH
- Stereotaxis, Inc.
- LivaNova PLC
- AliveCor, Inc.
- Imricor Medical Systems, Inc
- CardioFocus, Inc.
- Mayo Clinic Laboratories
- GeneDx (a subsidiary of Sema4)
- Invitae Corporation
- Nihon Medi-Physics Co., Ltd.
- Welch Allyn (a Hill-Rom subsidiary)
Recent Developments
- In February 2025, a discovery by researchers at Amsterdam UMC and Johns Hopkins University led to a new gene therapy for treating malignant cardiac arrhythmia, a condition responsible for a significant number of deaths worldwide. The gene, named SCN10a-short (S10s), was identified in the search for a one-off gene therapy that could improve heart function and prevent cardiac arrhythmias. S10s addresses the need to overcome reduced sodium current (INa) in heart cells, which is implicated in various arrhythmia conditions such as Brugada Syndrome and arrhythmias related to heart attacks or heart failure.
(Source: https://www.insideprecisionmedicine.com) - In May 2022, a late-breaking study found epicardial ablation in Brugada syndrome (BrS) helped significantly reduce sudden cardiac arrest in these patients, increasing their survival. The results of the Epicardial Ablation in Brugada Syndrome to Prevent Sudden Death trial was presented at Heart Rhythm 2022, the annual meeting of the Heart Rhythm Society (HRS).
(Source: https://cardiovascularbusiness.com)
Segments Covered in the Report
By Type of Treatment
- Implantable Devices
- Implantable Cardioverter Defibrillators (ICDs)
- Pacemakers
- Others
- Drug Therapy
- Antiarrhythmic Drugs
- Quinidine – Tier 1
- Isoproterenol
- Others
- Lifestyle & Behavioral Interventions
- Avoidance of Triggers
- Temperature Regulation
- Others
- Supportive & Emergency Care
- External Defibrillation Devices
- Emergency Response Kits
- Others
By Diagnosis & Monitoring
- Electrocardiogram (ECG)
- Resting ECG
- Provocative Drug Challenge Test (Ajmaline/Flecainide)
- Electrophysiological (EP) Testing
- Genetic Testing
- SCN5A Mutation Detection
- Others
By End User
- Hospitals
- Cardiology Clinics
- Ambulatory Surgical Centers
- Academic & Research Institutions
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting